Photosynthesis Research in the Hastings Lab
Photosynthesis is a fundamental biological process through which plants, algae and some bacteria convert light energy into chemically useful forms. In all organisms photo-conversion is achieved by the generation and stabilization of a radical ion pair between protein bound cofactors. That is to say that solar photon absorption results in the transfer of an electron from a specialized donor molecule(s) to an acceptor. Secondary and tertiary electron transfers further increase the distance between the donor and acceptor which in turn stabilizes the generated radical, preventing wasteful recombination reactions. Generation and stabilization of radical ion pairs occurs in a specialized pigment-protein complex called a reaction center. Figure 1 shows a schematic of the organization of the pigments involved in electron transfer in purple bacterial and photosystem I reaction centers. The time constants for electron transfer to the various pigments is also shown in figure 1.
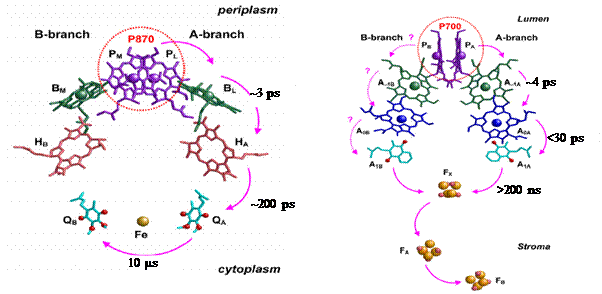
Figure 1: Cofactor arrangement in purple bacterial reaction centers (PBRCs) from R. sphaeroides (left), and in cyanobacterial photosystem I (PS I) from S. elongatus. In PBRCs the primary electron donor is a dimeric bacteriochlorophyll-a (BChl-a) species called P870. Upon light excitation of P870, an electron is transferred from P870* to HA (bacteriopheophytin-a) within ~3 ps. From HA¯, the electron is then transferred to QA in ~200 ps, and then to QB in microseconds. In PS I, following light excitation of the primary donor (P700), an electron is transferred to A0, a chlorophyll-a (Chl-a) acceptor, in ~4 ps. Stabilization of the charge-separated state is achieved by a second ET process; From A0¯ the electron is transferred to A1, a phylloquinone (PhQ) molecule in ~21 ps.
Reaction centers are truly remarkable entities in that they can convert solar photons into stable, chemical products with virtually perfect efficiency. One goal of research in my lab is to apply time-resolved and static infrared (IR) difference spectroscopy to study electron transfer (ET) cofactors involved in solar conversion processes in plants, algae, bacteria and artificial mimetic systems. Time-resolved Fourier transform infrared (FTIR) difference spectroscopy is poorly developed in the realm of photosynthesis, and presents attractive opportunities for interdisciplinary biophysical research.
All experimental research in my lab is complemented with computational simulatiosn, especially QM/MM vibrational frequency calculations for pigments bound to protein complexes. Specific areas in which I focus are described below.
Experimental: FTIR difference spectroscopy for the study of electron transfer cofactors in photosynthetic reaction centers.
1 Time-resolved FTIR difference spectroscopy for the study of the phylloquinone electron acceptor, A1, in photosystem I.
2 Static FTIR difference spectroscopy for the study of primary electron donors in photosynthetic reaction centers.
Photosystem I Type Donors: P700, P740, P798, P840
Photosystem II Type Donors: P680, P870, P960
3 Polarized, Infrared Micro-crystallography For Structural Studies of Cofactors In Photosynthetic Systems.
4 Far Infrared Detector Technologies for FTIR Difference Spectroscopy.
Computational: Calculation of the chemical properties of electron transfer cofactors in photosynthetic reaction centers
Density functional theory based methods for the calculation of the chemical properties of:
Phylloquinone and the A1 binding site in photosystem I
Ubiquinone and the QA binding site in purple bacteria
Plastoquinone and the QA binding site in photosystem II
Monomeric Chlorophyll's
Dimeric Chlorophyll's
P700, the primary electron donor in photosystem I
P680, the primary electron donor in photosystem II
P870, the primary electron donor in Rb. sphaeroides purple bacterial reaction centers